NGB-CTM
TRANSFORM YOUR RESEARCH IN THERAPEUTIC APPLICATIONS
With NGB-C™, the first clinical grade bioprinter in the world
One step further in the production of Advanced Therapy Medicinal Products
Accelerate patients’ access to innovative therapies with a totally reliable and reproducible GMP bioprinter
Exclusive laser bioprinting head
Discover high resolution and control precisely your cells patterns in 3D
Bioextrusion heads (4-60°C) & UV lamp (365 or 405nm)
Combine LAB with extrusion and a UV photo polymerisation head in a single equipment
GMP Multi-cellular microfluidic cartridge
Print multi-cellular tissue constructs with microfluidic cartridge and automated pipette. It includes multicolor cellular printing compatible with 3 different bioinks
GMP In-line cellular-level microscope (2μm resolution, 1 objective, 10×12 mm FOV) & ViewPrint software™
Get stunning images and monitor your printing process without taking tissues out of the sterile cabinet

Touch screen display with user friendly interface
Upload your SOPs and easily control tissue production
High-end GMP 6-axis robotic arm
Enjoy unrivaled precision & automation with fully-motorized robotics designed for sterile environment
Class A safety cabinet level
Contains an integrated air filtering system (2 blowing + 2 aspiration fans) to ensure a totally aseptic area
Do you want to know more about NGB-C™ ?
A unique and innovative LAB technology
Create highly reproducible patterns using different cell types and viscosity,
always with a very high cell viability !
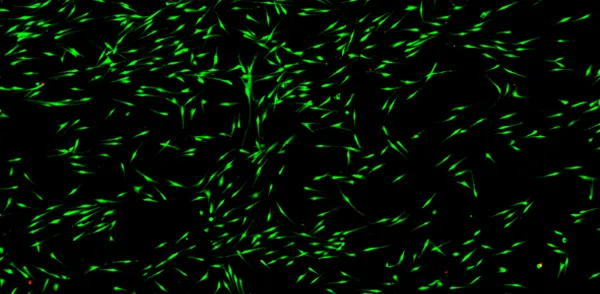
High cell viability
<95% cell viability
Print cells with a nozzle-free technology which doesn’t harm, damage or injure them throughout the bioprinting process.
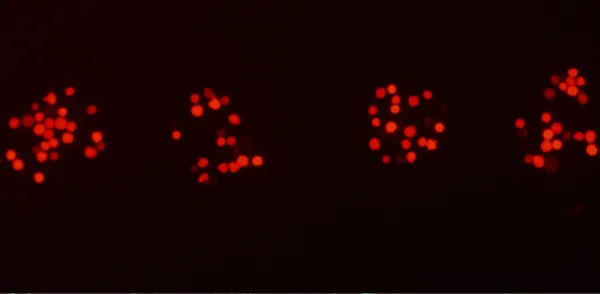
High resolution
10 µm resolution
Adjust printing resolution by playing with cell concentration on the donor and with the laser beam energy level.

High flexibility
From 20 to 300 mPa.s min
Print an important variety of bio-inks at different viscosities, cell types and materials as well as aggregates.
Do you want to know more about Poietis’ patented LAB technology ?
Discover our bioprinting solution
Poieskin®, the first therapeutic
bioprinted product
Bioprinted with NGB-C™, a completely GMP compliant high-end bioprinter
Poieskin® is an autologous dermal-epidermal skin substitute printed by NGB-C™ and indicated for permanent coverage of skin defects.
In 2020, Poietis signed a clinical research collaboration contract with the Assistance Publique – Hôpitaux de Marseille (AP-HM, France) to prepare the first clinical trial of a bioprinted skin.
In July 2023, this collaboration led to the qualification of NGB-C™ bioprinting platform in a GMP facility and the production of a 40 cm2 GMP-compliant bioprinted graft equivalent to the standard of care, skin autografting.
You can find here, the article of this in vivo proof of concept of a large-size bioprinted dermo-epidermal substitute for permanent wound coverage.

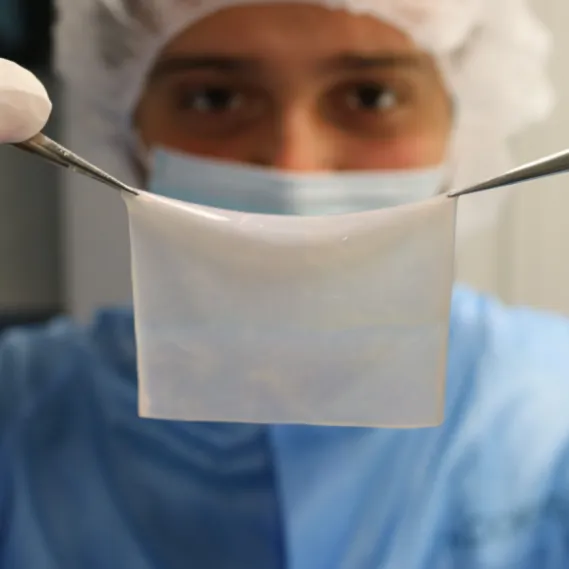
Poieskin® in clinical trials
Poieskin® biofabrication benefits from the latest advances in 3D bioprinting technology.
The high precision and resolution of Poietis’ bioprinter NGB-C™ as well as the embedded in-line monitoring systems, enable to control the quality of each bioprinted layer and hence to manufacture controlled 3D cell structures and reproducible tissue models.
The conclusion of the Poieskin® proof of concept underlines the fact that Poieskin® is reliable and homogeneous, leading to a clinically satisfying rate of graft taken compared to the reference human STSG in a mouse model.
Based on these results which confirm that Poieskin is compatible with pharmaceutical guidelines, a phase I/IIa clinical trial is in preparation.

Bioprinted skin prepared from a simple sample of the patient’s own cells would greatly simplify the surgical procedure and avoid large skin samples. This therapeutic innovation « made in France » could be a real revolution in the treatment of patients requiring a skin graft, such as severe burns.
Dr. Bertrand, plastic surgeon at the AP-HM (Marseille, FRANCE)

This collaboration will bring together all the skills required to produce a bioprinted tissue that can be implanted in humans, in accordance with European regulations. But this is only one step. Tomorrow, the objective is to bioprint more complex tissues as close as possible to the patient. This reminds me of the success of surgical robotics: a breakthrough innovation that has become essential in daily practice.
Dr. Magalon, pharmacist-biologist in the Cellular Therapy Unit at the AP-HM (Marseille, FRANCE)
Interested in NGB-C™ advanced bioprinting platform ?
Let’s talk about your printing needs, our experts are available to support you !
